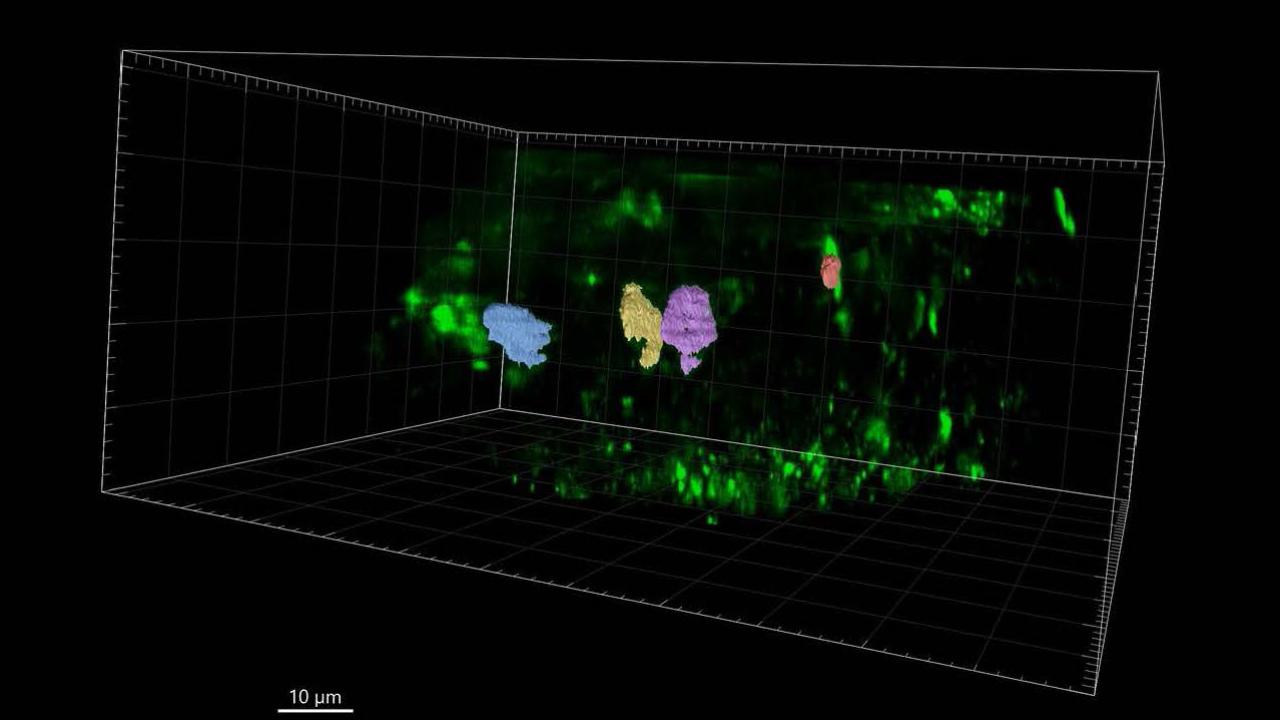
Sinclair shows three phases of building a cell wall using 4D imaging
Plant cell division shifts from accumulation to recycling, Drakakaki lab proves
If you put a cutting of, say, a geranium or an inchplant, into a cup of water, you can see tender new roots reaching out within a few days. Soon, a tiny new leaf pokes out of the stem. What you’re seeing is the result of cell division – without which, nothing grows.

When plants grow, they build new cells in a process that’s akin to dividing a room. For the first time, UC Davis researchers have uncovered how the pieces of the new wall come together and the timing of the construction. Their work, which was recently published in the Journal of Experimental Botany, offers a guidepost for other scientists who are untangling the basics of plant cell division.
“We knew the components that go into building the new cell wall, but not how they come together,” said Rosalie Sinclair, the first author on the paper and a recent doctoral degree graduate in the Department of Plant Sciences. “This was the first time we were able to watch it happen live, start to end, in a living sample.”
Back to dividing a room: When people build the new wall down the middle, they bring in materials – for our example, we’ll say bricks and cement. They need wheelbarrows and trowels, shovels and scaffolding and plumb lines. Workers erect the wall, then they go away, and they take the tools and scaffolding and extra material with them, leaving a clean wall and two new rooms where once there was one.
In plants, this is the last phase of cell division, and it’s called cytokinesis. Understanding cell wall construction is basic for understanding plants, said Georgia Drakakaki, a professor in the department and principal investigator in the research. “Without cell division, there is no plant life,” she said, and without plants, no life as we know it.
Sinclair, Drakakaki and team made the discoveries using a new type of microscope, which allowed them to look at plant cells and record the cell division process in four dimensions, including time. “It is like a CT scan,” Drakakaki said, motioning over her body. “It takes images at multiple time points throughout the cell division process.” It also vastly speeded up the work of gathering the data.
“It opens the path for a very complex process to be analyzed in great detail in a simple way,” Drakakaki said.
Three phases of building a new cell wall
In another first, Sinclair and team were able to measure the amount of membrane material delivered to the building site and how that amount changes over time. They also identified the critical point when a complex sugar called callose is deposited at the building site, and determined its key role in building the cell wall. That led them to discerning three distinct phases of the construction process: Large amounts of cytokinetic vesicles – think of them as the wheelbarrows carrying the building material -- appear in the cell, gather to form the new wall, and then quickly disappear.
“We found there’s a big volume of material that’s not used,” Drakakaki said. The cell recycles that unused material, and although scientists had postulated recycling takes place, “we didn’t know the timing of when that started, or that it was so much,” added Sinclair. “We found that the material recycling starts about 10 minutes after the cell plate assembly has started.”
At that critical point, they found that callose – think of it as temporary cement -- is necessary to stabilize the new cell wall until the construction is finished.
The team also developed a new way to analyze the large volumes of data they had collected, creating a new methodology with benchmarks for comparison, based on cytokinetic vesicle volumes rising and falling. Other researchers can use these benchmarks and methodology to study other elements used in cell wall formation.
Cutting-edge microscope gives new view – including time

The team made the breakthroughs using a new type of microscope that lets them see plant cells dividing from start to finish and record images in four dimensions. It is the first time lattice light sheet microscopy has been used in plants to this extent, Drakakaki said. “Our study may encourage the scientific community to see the value of this trail-blazing approach,” she added.
Drakakaki and fellow researcher Thomas Wilkop, in the UC Davis Light Microscopy Imaging Facility, used the LLS microscope to gather their data, with the help of teammates and equipment at the Janelia Research Campus in Ashburn, Va.
The new microscope “vastly improves the combined spatial and temporal resolution in the imaging,” Wilkop said: In 2016, Drakakaki sought similar information using a confocal laser scanning microscope, which is more commonly available to researchers. Working over the entire summer, she collected a tiny fraction of the data they obtained in a single day with the LLS microscope.
Related links
Team members in the Drakakaki lab include Minmin Wang and Toshisangba Longkumer Chuba.
Read the paper by Sinclair, Drakakaki and team in the Journal of Experimental Botany: “Four-dimensional quantitative analysis of cell plate development in Arabidopsis using lattice light sheet microscopy identifies robust transition points between growth phases.”
Watch a fun animated video, also from the Drakakaki lab, that shows how plant cells divide, here.
Media Resources
- Trina Kleist, UC Davis Department of Plant Sciences, tkleist@ucdavis.edu, (530) 754-6148 or (530) 601-6846
